Kombucha Code of Practice
Kombucha Brewers International
Code of Practice
Version 3.0
Product Standards & Food Safety Requirements
Product Scope: This standard applies to all beverages bearing the name of kombucha, from concentrate or not, flavored or not, intended for direct consumption in conformity with the definitions of this Code of Practice. Kombucha Brewers are required to comply with the standards listed in order to pursue certification.
The purpose of this code of practice is to create a food safety and quality standard for kombucha producers that creates transparency for consumers to make informed choices. The certification and seal program provide a high level of trust for food safety and an understanding that the product follows clear standards for kombucha manufacture.
Table of Contents
- Kombucha Product Standard
- Processing and Ingredient Definitions
- Finished Product Definitions
- Kombucha Brewing Process Steps
- Kombucha Brewing Process Flow Chart
- General Hygiene Requirements
- Kombucha Labeling Requirements
- Analytical Profile of Kombucha
Kombucha Product Standard
- Kombucha Product Standard
Kombucha tea is a slightly sweet/acidic fermented beverage made with an aqueous extract of tea leaves (fermented) utilizing a symbiotic culture of bacteria and yeast (SCOBY or pellicle). Kombucha typically has a pH range of 2.3 to 3.8. The fermentation produces a beverage with some natural carbonation, organic acids, nutrients in natural form and trace amounts of alcohol. Variations in the base solution and optional ingredients, as well as further possible fermentation processes provide a variety of related similar products with different flavor profiles. Sanctioned variations are listed in this standard.
Processing and Ingredient Definitions
2. Processing and Ingredient Definitions
2.1 Tea: An unsweetened beverage obtained from soaking the leaves of the Camellia sinensis plant in water. Same as Tea Liquor. Variations of Camellia sinensis include but are not limited to black, green, white, oolong, yellow, pu-erh and so forth.
2.2 Tea liquor: The liquid from steeped tea leaves, same as brewed tea.
2.3 Sweetened tea liquor: Steeped tea with an added nutritive carbohydrate.
2.4 SCOBY: An acronym that stands for “Symbiotic Culture of Bacteria and Yeast”. The SCOBY contains a mixture of Acetic Acid Bacteria (AAB) species and any of a number of yeast species including but not limited to Brettanomyces bruxellensis; Zygosaccharomyces; Candida spp. and so forth that will vary based on region. It manifests in two microbiologically distinct phases. Either or both phases may be used as inoculant. See Analytic Profile of Kombucha.
2.4.1 Liquid Phase SCOBY: Commonly referred to as “starter liquid” or “backslop,” it refers to already fermented kombucha from the previous batch. It may also be independently produced to yield a lower pH and lower Brix for a variety of reasons including speeding up the fermentation process and lowering the pH more quickly.
2.4.1.1 It is microbiologically active and capable of producing solid phase SCOBY.
2.4.1.2 The amount of liquid phase SCOBY to be used, especially if no solid phase SCOBY is employed, is typically no less than 10% of the total batch volume to ensure product safety.
2.4.2 The solid phase of SCOBY is commonly referred to as pellicle, bacterial cellulose and mother culture. It is a matrix of bacterial cellulose and yeast used as inoculant.
2.4.2.1 The solid phase requires liquid phase to be present for best results.
2.5 Nutritive carbohydrate sweetener: Any sugar source that facilitates the fermentation process including but not limited to sucrose, fructose, glucose, galactose, dextrose, lactose, maltose, as well as other nutritive carbohydrate sweeteners that support fermentation such as honey, agave, molasses, brown sugar, maple syrup and certain fruit juices. Must be a type that will ferment to provide the characteristics of traditional kombucha.
2.6 Kombucha Base: An intermediary product not sold directly to consumers. Kombucha fermentation techniques as specified in the standard must be employed to achieve the base which typically has been fermented so that the sugars end up at 0%, and acetic acid concentration is over 1.2%. This product cannot be consumed without dilution and is considered a manufacturing sub-ingredient for the finished product. It requires dilution of over 20% of water, juice, tea or other liquid to produce a safe and palatable kombucha-style product and is therefore not subject to the labeling requirement. Kombucha base must be indicated as “from concentrate” on the front of the label as well as in the ingredients panel. See Labeling Requirements.
2.6.1 Kombucha Base that is produced through traditional, long term fermentation (30 days or longer) may contain a greater quantity and variety of acids and nutrients based on HPLC testing.
2.6.2 Kombucha base which is produced in a shorter time using techniques such as fermentation temperatures that exceed those listed in section 4.3, forced oxygenation, constant stirring or movement of the liquid from one tank to another, and so forth. It is still subject to the same labeling requirements as 2.6.
Finished Product Definitions
3. Finished Product Definitions
3.1. Traditionally Fermented Kombucha Tea: A traditional beverage that is obtained from the fermentation of various types of tea leaves, sugar, SCOBY, and starter liquid which results in a tangy, slightly sweet, effervescent liquid with low levels of alcohol and without additional heat treatment after fermentation. Flavoring may be added. his kombucha will grow a spontaneous SCOBY if undisturbed in an open container at temperatures between 70º-90ºF (21º-32ºC)
3.1.1 Mandatory Ingredients:
3.1.1.1. Camellia sinensis leaves
3.1.1.2. Potable Water
3.1.1.3. Nutritive carbohydrate sweeteners (See Labeling Requirements)
3.1.1.4. Liquid and solid phase SCOBY
3.1.2. Optional Ingredients (not to exceed 20% of the finished product by volume formulation solely or in combination) See Labeling Requirements
3.1.2.1. Flavorings ingredients including various juices
3.1.2.2 Characterizing food ingredients
3.1.2.3. Carbon dioxide
3.2. Kombucha: A beverage obtained from fermentation of any number of acceptable plant materials, SCOBY/starter liquid, water and a fermentable sugar. Flavoring may be added. Depending on processing steps, this kombucha may or may not grow a SCOBY spontaneously.
3.2.1. Mandatory Ingredients:
3.2.1.1. Camellia sinensis, coffee, yerba mate, herbs or other defined substrate per Finished Product Definitions
3.2.1.2. Potable Water
3.2.1.3. Nutritive carbohydrate sweeteners
3.2.1.4. Liquid and/or solid phase SCOBY
3.2.2 Optional Ingredients (not to exceed 20% of the finished product by volume formulation solely or in combination) See Labeling Requirements
3.2.2.1. Flavorings ingredients including various juices
3.2.2.2. Aroma and flavor-producing microbial cultures
3.2.2.3. Vitamins and minerals
3.2.2.4. Characterizing food ingredients (such as chia seeds)
3.2.2.5. Probiotic bacteria
3.2.2.6. Carbon dioxide
3.2.2.7. Citric and other acids
3.2.2.8. Color additives
3.2.2.9. Stabilizers – used only in flavored, colored or vitamin and mineral fortified kombucha
3.3. Jun Kombucha: A traditional kombucha, except that only raw honey may be used as a sweetener
3.4. Honey Kombucha: A traditional kombucha, except that only pasteurized honey may be used as the sweetener.
3.5. Herbal Kombucha: A traditional kombucha, except that a minimum of 20% of the plant material is composed of herbs.
3.6. Coffee Kombucha: A traditional kombucha, except that a minimum of 20% of the plant material is composed of coffee.
3.7. Yerba Mate Kombucha and Yaupon Kombucha: A traditional Kombucha, except that a minimum of 20% of the plant material is composed of yerba mate or yaupon.
3.8. Hard Kombucha: A kombucha to which which is crafted to yield a higher alcohol content than traditional kombucha using non-native yeasts or combining with higher alcohol containing beverages such as beer, cider, wine, spirits, etc. These products contain an alcohol content greater than 4% ABV and are subject to local excise taxes.
3.8.1. Crafted Hard Kombucha: A traditional kombucha fermentation to which additional non-native yeast is added at a later phase resulting in a higher than possible alcohol content.
3.8.2. Spiked Hard Kombucha: A kombucha to which alcohol is added to yield a higher alcohol beverage. Could also be termed “Kombucha Cocktail.”
Kombucha Brewing Process Steps
4. Kombucha Brewing Process Steps
4.1. Tea leaves (and/or coffee, herbs, yerba mate, etc.) are steeped in water and then removed to produce tea liquor.
4.1.1. Hot steep or cold steep is acceptable provided it meets the safety requirements of your local regulators.
4.1.2. Steeping time will vary by producer and type of substrate used.
4.1.3. Other botanicals, fruit or ingredients may be added at this stage of the fermentation process.
4.2. The tea liquor is mixed with a nutritive sugar source.
4.3. Once the sweetened tea liquor is at the right temperature for the SCOBY, then it is added.
4.3.1. The amount of SCOBY may differ between kombucha manufacturers and in different regions of the world though a minimum of 10% is required for food safety reasons.
4.3.3. pH is recommended to be 4.6 or lower after adding the SCOBY.
4.4. Fermentation continues at the desired temperature in an aerobic environment for an established time.
4.4.1. The fermenting substrate will start to smell acidic with evidence of gas bubbles appearing.
4.4.2. A new SCOBY will start forming across the surface of the liquid concurrent with an anaerobic fermentation process to occur below the surface of the liquid.
4.4.3. The taste of the kombucha changes during fermentation from a pleasantly fruity, tangy flavor after a few days to a vinegary taste after a longer fermentation time.
4.4.4. The color of the liquid will also lighten the longer it is fermented.
4.4.5. Some may choose to use closed tank systems and bubble in air/oxygen.
4.4.6. Kombucha base is typically subject to rapid fermentation in closed tank systems using oxygenation techniques common in the vinegar making industry.
4.5. Fermentation time will vary based on several factors including size and shape of vessel, temperature & humidity, type of substrate, amount of SCOBY added and so forth.
4.6. At the end of primary fermentation, the newly formed cellulosic pellicle is removed and may be used for future fermentation provided no signs of mold are present.
4.7. A flavor infusion step may occur at this point in the process and if there are fermentable sugars present in the flavorings, then would be considered secondary fermentation.
4.8. A tertiary fermentation may occur if the product is permitted to bottle age; that is remain bottled for a period of time to induce flavor changes and/or changes in natural effervescence.
4.9. At any stage, the beverage may be filtered to remove excess yeast.
4.9.1. Filtration equal to or less than 0.45 microns, must be indicated on the label as “sterile filtered”. See Labeling Requirements
4.10. The finished product is then packaged and stored at refrigeration temperatures between 40ºF (4.4ºC) – 48ºF (9ºC) throughout its distribution chain, to inhibit additional fermentation that could lead to increased alcohol content, reduced shelf life and off flavors. Exact temperature may vary based on local regulations.
4.10.1. If the product is pasteurized or otherwise subjected to processing steps to create a shelf stable product, then refrigeration is not needed.
4.10.1.1. Other shelf stable step include but are not limited to:
4.10.1.1.1. High pressure pasteurization
4.10.1.1.2. Chemical sterilization or pasteurization
4.10.1.1.3. Sterile filtration
4.10.2. Kombucha that is kegged must utilize equipment and practices as outlined in the Kombucha Draught Standards Manual.
5. Additional Processing Steps
5.1. Some Kombucha tea undergoes further processing that could include:
5.1.1. Secondary fermentation naturally produces carbon dioxide and ethanol which adds carbonation and varying levels of “fizz” to the Kombucha beverage.
5.1.1.1. Kombucha undergoing natural secondary fermentation may be indicated on the label as “naturally effervescent” or “naturally carbonated” assuming no form of forced carbonation or chemical reaction to produce additional carbonation is used.
5.1.1.2. Kombucha undergoing natural secondary fermentation needs to confirm ethanol levels are in compliance for their region. See Alcohol Chart below.
5.1.2. Pasteurization at any stage to kill organisms and limit further fermentation in the bottle.
5.1.2.1. Pasteurized Kombucha must be indicated on the label. See Labeling Requirements
5.1.3. Dealcoholized: Any technique, mechanical, chemical or otherwise employed after fermentation to remove all naturally occurring ethanol from the product.
5.1.3.1. Kombucha that has had some or all naturally occurring ethanol removed must have this indicated on the label. See Labeling Requirements
5.1.4. Dilution: Kombucha may be diluted with water or juice after fermentation and prior to bottle.
5.1.4.1. Dilution % must be indicated on the label only if it contains more than 20% of added ingredients and is not a product made using kombucha base.
5.1.4.2. Juice % must be listed above the nutrition panel in accordance with 21 CFR 101.30.
5.2. Kombucha can be produced using a kombucha base provided the base was produced in the same manner as listed in steps 4.1-4.6 above.
5.2.1. Kombucha produced from base must have this indicated in the ingredients list as the product “Kombucha base (INGREDIENTS BY WEIGHT IN THE BASE)” and also indicate on the front of the label that the product is “from concentrate.” See Labeling Requirements
Kombucha Brewing Process Flow Chart
Exact steps will vary by producer
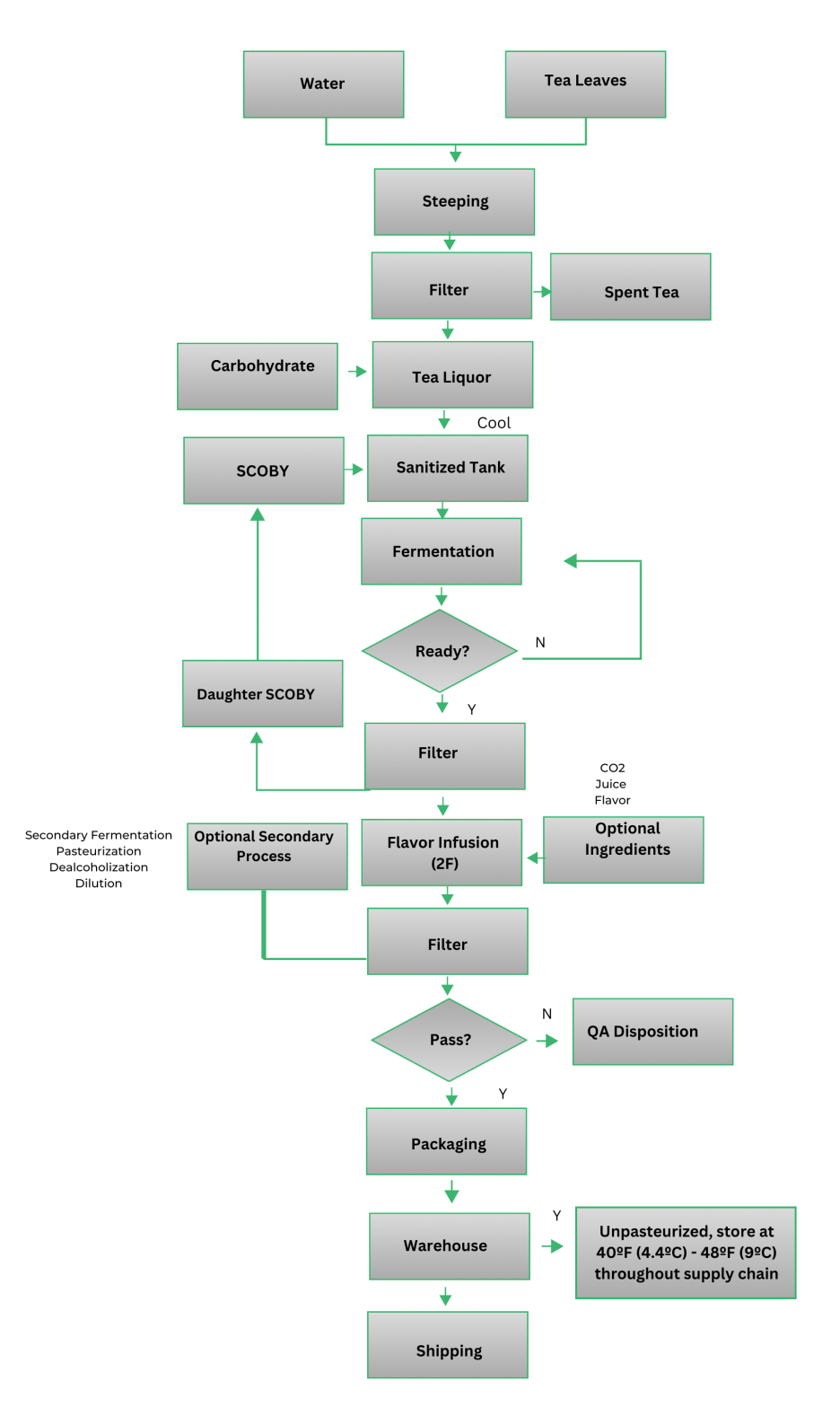
General Hygiene Requirements
7. General Hygiene Requirements
7.1. Primary Production: Primary production should be managed in a way that ensures that food is safe and suitable for its intended use. Where necessary, this will include:
7.1.1. Avoiding the use of areas where the environment poses a threat to the safety of food.
7.1.2. Controlling contaminants, pests and diseases of animals and plants in such a way as not to pose a threat to food safety.
7.1.3. Adopting practices and measures to ensure food is produced under appropriately hygienic conditions.
7.2. Establishment Design and Facilities: Depending on the nature of the operations, and the risks associated with them, premises, equipment and facilities should be located, designed and constructed to ensure that:
7.2.1. Contamination is minimized.
7.2.2. Design and layout permit appropriate maintenance, cleaning and disinfections and minimize airborne contamination.
7.2.3. Surfaces and materials, in particular those in contact with food, are non-toxic in intended use and, where necessary, suitably durable, and easy to maintain and clean.
7.2.4. Where appropriate, suitable facilities are available for temperature, humidity and other controls.
7.2.5. Effective protection against pest access and harborage.
7.3. Control of Operation: To produce food that is safe and suitable for human consumption.
7.3.1. Formulate design requirements with respect to raw materials, composition, processing, distribution and consumer use to be met in the manufacture and handling of specific food items
7.3.2. Design, implement, monitor and review effective control systems.
7.4. Establishment: Maintenance, Sanitation and Personal Hygiene
7.4.1. Ensure adequate and appropriate maintenance and cleaning.
7.4.2. Control pests.
7.4.3. Manage waste.
7.4.4. Monitor effectiveness of maintenance and sanitation procedures.
7.4.5. Ensure that those who come directly or indirectly into contact with food are not likely to contaminate food.
7.4.5.1. Maintain an appropriate degree of personal cleanliness.
7.4.5.2. Behave and operate in an appropriate manner.
7.5. Transportation: Measures should be taken where necessary.
7.5.1. Protect food from potential sources of contamination.
7.5.2. Protect food from damage likely to render the food unsuitable for consumption
7.5.3. Provide an environment that effectively controls the growth of pathogenic or spoilage microorganisms and the production of toxins in food.
7.6. Product Information and Consumer Awareness: Products should bear appropriate information (i.e. lot coding).
7.6.1. Ensure adequate and accessible information is available to the next person in the food chain to enable them to handle, store, process, prepare and display the product safely and correctly
7.6.2. Easily identify the lot or batch and recall if necessary.
7.7 Recall Procedure: Sometimes raw kombucha can continue to ferment in the bottle and move out of compliance for alcohol due to natural fermentation from supply chain issues or temperature abuse. The vast majority of these products are still safe to consume, however to be in compliance, they may need to be removed from the point of sale.
7.7.1. Any product deemed subject to recall in the United States is subject to the FDA Regulatory Procedures Manual; Chapter 7 Recall Procedures
7.7.2. Any product deemed necessary to recall in any other country is subject to their government agencies recall procedures. See chart in Labeling Requirements
Kombucha Labeling Requirements
8. Labeling Requirements: The name of the beverage that meets all of the above requirements in this industry code of practice is either “Traditionally Fermented Kombucha Tea” or “Kombucha” or any of the Finished Product Definitions assuming the product conforms to the specifications for that definition.
8.1. The following terms shall accompany the name wherever it appears on the principal display panel or other panels of the label in letters not less than one-half of the height of the letters used for the name of the food.
8.1.1. The term “Pasteurized” if pasteurized. Must meet 21 CFR part 117 requirements.
8.1.2. The phrase “From Concentrate” if produced using a kombucha base.
8.1.2.1. In addition to being visible on the front panel, it also needs to be listed on the ingredients label in the order from greatest to least thusly: Water, Kombucha base (INGREDIENTS BY WEIGHT IN THE BASE), flavorings, stevia, etc.
8.1.3. The phrase “sterile filtered” if passed through a filter of equal to or less than 0.45 microns.
8.1.4. The phrase “preservatives added” if chemically pasteurized. Preservatives must be listed in the ingredients statement.
8.2. The following terms shall appear on the label in letters consistent with ingredient panel label requirements.
8.2.1. If naturally occurring ethanol was removed prior to bottling, it must be noted on the label.
8.2.2. If carbon dioxide is infused just prior to or during bottling, it must be noted on the label. This must be listed in the ingredient statement.
8.2.3. The word “X% kombucha” must be stated if the product has less than 80% kombucha and is not made from kombucha base.
8.2.4. The phrase “Added cultures” if additional bacteria or yeast are added prior to bottling. These must be listed in the ingredient statement.
8.2.5. The phrase “Added probiotics” if probiotics are added. These must be listed in the ingredient statement.
8.2.6. The phrase “Added acids” if additional organic acids are added. These must be listed in the ingredient statement.
8.2.7. The phrase “Flavored with XX” and/or “Colored with XX” with the “XX” identified as specific flavoring or coloring agent. These must be listed in the ingredient statement.
8.2.8. The phrase “sterile filtered” must be included on any products that are passed through a filter of equal or less than 0.45 microns.
8.2.9. A refrigeration statement may be required if the product is not subject to processing steps that eliminate the need for refrigeration such as pasteurization, sterile filtration, etc.
8.2.9.1. Examples include: “Keep Refrigerated” or “Store Refrigerated.”
8.2.10. Ethanol labeling may be required. See Ethanol Compliance
8.2.11. When listing ingredients, if sugar is used as the nutritive carbohydrate source, then final sugar grams must be listed as “Added Sugars.” This applies to kombucha produced in the United States. Labeling requirements for sugar may vary based on country.
8.2.12. When listing ingredients, the term “Kombucha Cultures” may be used provided the cultures used conform to the properties listed in section 10.5.
8.3. The following marketing terms may be used if desired.
8.3.1. The phrase “Naturally Effervescent” if no artificial carbonation has been injected prior to or during bottling.
8.3.2. The phrase “Wild Culture” if using a starter liquid and/or SCOBY that has been cultivated from wild strains and has not been designed nor bioengineered. The culture may be manipulated through hybridization or natural cultivation – i.e. desired characteristics cultivated through selective breeding.
8.3.3. The phrase “Raw” if the kombucha has not been subjected to pasteurization, sterile filtration or chemical sterilization and is able to spontaneously produce a SCOBY if left at the right conditions.
8.3.4. The phrase “Cold Filtered” if the kombucha has been filtered via temperature.
8.3.5. Bespoke/Lab Created Cultures: Cultures of limited strains of AAB and any number of yeasts native to kombucha that are generated in a lab and intended to create specific outcomes – ie reduced alcohol content, flavor profile. These cultures do not contain the same diversity of species as a wild culture and may or may not confer the same health outcomes to consumers.
8.3.6 The term “Unfiltered” may be used for kombucha that has not been filtered which creates a different flavor and mouthfeel due to the presence of yeast.
8.4. When kombucha meeting this Code of Practice is mixed with another type of beverage or food, the result is a compound food and the percentage of Kombucha in the finished beverage must be identified, i.e.“Contains _ percent (or %) Kombucha” or a similar phrase, with the blank filled in with the percentage expressed as a whole number not greater than the actual percentage of kombucha. The percentage of kombucha declaration shall be placed prominently on the primary display panel, in easily legible boldface print or type in distinct contrast to other printed or graphic matter.
Kombucha Specifications
9. Kombucha Specifications
9.1. Kombucha shall be practically free from contaminants and meet all regulatory requirements for human food.
| Contaminant | Maximum limit | Method of test |
| Arsenic (As), mg/kg | 0.05 | US ISO 6634/NF EN 15763 |
| Lead (Pb), mg/kg | 0.05 | US ISO 6633 |
| Mercury (Hg), mg/kg | 0.001 | US ISO 6637 |
| Cadmium (Cd), mg/kg | 0.003 | US ISO 6561-2 |
9.2. Kombucha shall not contain vinegar eels nor fruit flies.
Analytical Profile of Kombucha
10. Analytical Profile of Kombucha
10.1 Chemical Standards
| Substance | Source | Concentration | Range | Comments |
| Alcohol (ETOH) | Finished product | In compliance with local regulations See chart below for additional details by country & region. |
0.00-3.2%ABV | Ethanol in kombucha naturally ranges from 0-3% ABV |
| Titratable Acidity
-Acetic Acid |
Finished product | Max 2.0% | 0.27-2.03% | |
| pH | Finished product | Max 3.8 | 2.3-3.8 | Must be less than 4.6 for food safety |
10.2. Ethanol Compliance
10.2.1. At a minimum, send to a 3rd party lab on annual basis to confirm food safety and compliance. Local regulators may require more frequent ethanol testing by third parties.
10.2.1.1. Labs and their testing methods vary greatly. It is highly suggested that a ISO 17025 accredited or similarly reliable lab be used. In lieu of an accredited lab, it is imperative to understand the testing methods to confirm they are accurate for ethanol in kombucha.
10.2.2. Retain on file lab results which demonstrate an alcohol level in compliance for your country and region.
10.2.3. A minimum of twice per year, the company should test its kombucha pulled from retention samples held in house, and must maintain records that demonstrate that the alcohol level is below the legal limit for your country.
10.2.4. Non Alcoholic Kombucha: Must comply with local laws. See Chart below for reference.
10.2.4.1. Ethanol testing is recommended for every batch for food safety and compliance using a KBI Approved Ethanol Testing Method.
10.2.5. Hard Kombucha:
10.2.5.1. Determine whether the kombucha requires permits as a brewery or winery depending on your local regulations. Hard Kombucha may be classified as beer, wine or hard seltzer depending on the method and ingredients of manufacture.
10.2.5.2. Comply with all regulations regarding the best practices for a product of its classification.
10.3. Consult your legal team to determine what if any alcohol warning statement is required on your product.
10.3.1. Example Statements: “Kombucha is a fermented tea that has naturally occurring alcohol.” “Kombucha naturally contains trace amounts of ethanol due to the fermentation process.”
10.3.2. May also add language to the effect of “ Do not consume if you are avoiding alcohol.”
10.4. Ethanol Compliance Chart by Country (send any updates for your country/state or province to admin@kombuchabrewers.org)
| Country | Ethanol Level for All Ages | Ethanol Level for labeling | Ethanol level for taxation | Comments |
| United States | <0.5% | <0.5% | >0.5% | KOMBUCHA Act, when it passes, would change threshold for taxation to 1.25% ABV |
| Australia – New South Wales, Victoria, Queensland | <0.5% | >0.5% | ||
| Australia – Western Australia, South Australia, Tasmania | <1.15% | >1.15% | ||
| Canada – Alberta, British Columbia, Manitoba, | <1.1% | <1.1% | >1.1% | |
| Canada – Nova Scotia, Ontario, PEI, Quebec, Saskatchewan | <0.5% | <0.5% | >0.5% | |
| Europe – Belgium | <0.5% | |||
| Europe – Denmark, Lithuania, Luxembourg, Slovenia, Spain | <1.2% | <1.2% | >1.2% | |
| Europe – Finland | <1.2% | <1.2% | >2.8% | 1.2-2.8% pay taxes for alcohol but still considered a “non-alcoholic” beverage – NO marketing allowed for alcoholic beverages unless below 2.8% |
| Europe – Slovakia | <.75% | |||
| Mexico | <2.0% | |||
| New Zealand | <1.2% | |||
| South America – Colombia | <2.5% |
10.5. Microbiological Standards
| Microflora | Source | Range |
Bacteria: Acetic Acid Bacteria (AAB)
|
SCOBY | AAB dominant (more than 50%), may also contain some Lactic Acid Bacteria (LAB) in less significant quantities |
Yeast:
|
SCOBY | Yeast vary by region and contribute to flavor and ethanol content |
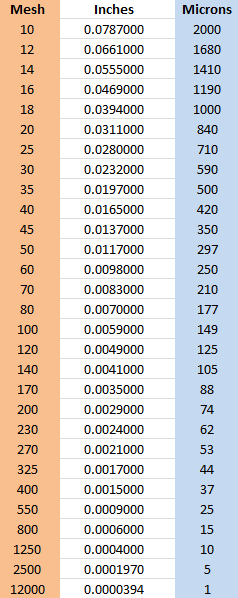
10.6. Mesh to Micron Comparison Chart
Last updated 5.18.23

