KBI Approved Ethanol Testing Methodology
Like all fermented foods, kombucha also produces trace amounts of alcohol typically ranging from 0.1-2% ABV depending on several factors such as brewing procedures/parameters, sugar content, storage conditions, etc. The trace amounts of ethanol serve multiple functions – as an antimicrobial to prevent mold; as a solvent to extra nutrients; and as a medium for easing absorption of nutrients by the consumer.
The trace amounts of ethanol in kombucha are not typically intoxicating as they are combined in a matrix of organic acids that neutralize the toxifying effects of alcohol. However, the laws for what is labeled an alcoholic beverage vary by country and sometimes state or province. Therefore accurate testing methods are crucial for ensuring compliant products.
Alcoholic Beverages Defined
According to Title 27 United States Code Chapter 8 – Federal Alcohol Administration Act Section 214, “The term “alcoholic beverage” includes any beverage in liquid form which contains not less than one-half of one percent of alcohol by volume and is intended for human consumption.”
Across the globe, regulations vary.
[table id=10 /]
Since kombucha may sometimes contain more than 0.5% ABV, some Kombucha breweries in the US have elected to brew their Kombucha under a state beer or wine license – the designation of which varies by state law. This is often referred to as “Over 21 Kombucha” or “AP5 Kombucha” and sometimes called “Traditional Kombucha” as most homebrews likely fall within this range.
Many commercial producers have chosen to tightly control their processes to remain non-alcoholic beverages using a variety of techniques including cone-distillation, microbial manipulation, filtration, and other known ethanol control techniques. The primary reason for doing so is to avoid the hassle of distributing alcoholic beverages which is more complex and varies state to state resulting in a complicated system that limits access to consumers. Also because kombucha is a health beverage, many prefer to keep it in the purview of other foods.
“Hard Kombucha” as a category emerged in 2010 and has gained increasing popularity in the last few years. Hard Kombucha requires non-native yeasts to be added to kombucha to yield higher alcohol content (4% ABV and higher) beverages that are subject to all Federal and local laws, labelling and taxation.
A few brands are producing offerings in multiple categories.
KBI Recommendation for Accurate Ethanol Testing: Headspace Gas Chromatography
The KBI Board of Directors current recommendation is that those seeking alcohol testing to demonstrate legal compliance utilize Headspace Gas Chromatography combined with Flame Ionization Detection (GC-FID) or Mass Spectrometry (GC-MS) from a third-party lab that employs methods specific for kombucha. This is based on the work of the AOAC Working Group (see below).
Headspace Gas Chromatography is the methodology used in the law enforcement field to determine blood alcohol levels as low as .01%, accuracy required for forensics that makes it the preferred choice for the regulatory agency.
In Headspace Gas Chromatography, the headspace (air volume) of a pure product creates an equilibrium when pressure is applied. Only a minute amount of sample, a tenth of a gram, is required to obtain test results.
- The sample is pressurized with helium then purged to remove all other volatile substances from the sample and captures them on a trap made of carbonaceous material.
- The trap is then desorbed or heated rapidly onto a Gas Chromatograph
- Then flame ionization detection (FID) or mass spectrometry (MS) is used to detect the alcohol level.
This method eliminates suspended and dissolved solids and removes any enzymes and bacteria that might interfere with the ‘gravity’ of the sample.
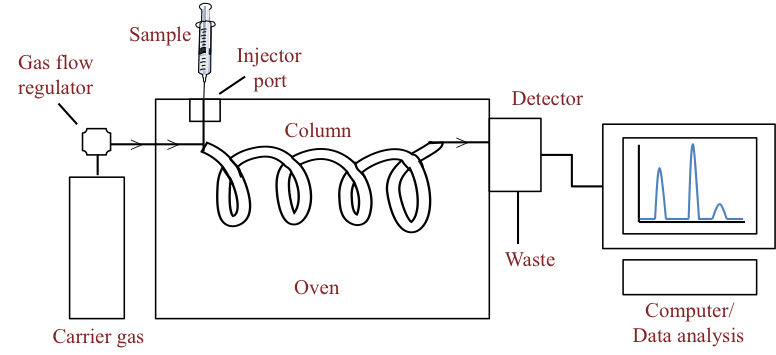
Gas Chromatograph Process Flow Diagram
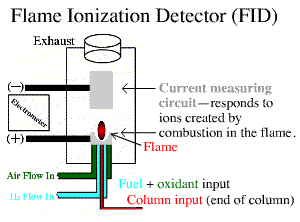
Flame Ionization Detection is a scientific instrument that measures analytes in a gas stream and is typically used as a detector in gas chromotography. “Flame ionization detectors are mass sensitive, meaning their measurements are proportional to the mass of the carbon that passes through them. FIDs operate by detecting ions formed during the combustion of organic compounds in a hydrogen flame. The generation of these ions is proportional to the concentration of organic species in the sample gas stream. Hydrocarbons generally have molecular response factors equal to the number of carbon atoms in their molecule. Molecules that contain only carbon and hydrogen respond best to flame ionization detection. As ethanol is primarily made up of hydrogen and carbon, it can be accurately analyzed by FID.” (source here)
Mass Spectrometry utilizes a fingerprint of known origin by establishing a calibration curve (5 point) using ethanol in water. A long-established sound scientific technique, this process produces results with 99% confidence level.
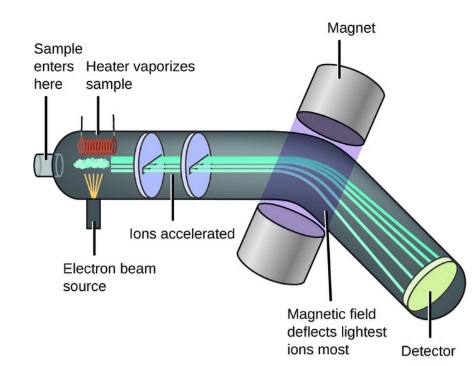
Mass Spectrometer Flow Diagram
Tips to Consider When Selecting a Lab Partner
- Question them thoroughly to gain an understanding of how the sample is handled to ensure the highest quality results
- How is the sample stored?
- At what temperature?
- Is the sample required to be sent in a full-size bottle or will a test tube suffice?
- Provide the lab with the handling protocol (see pdfs of methods linked below) & confirm they are able to execute the steps as outlined.
- Insist that higher-level technicians complete the test to ensure accuracy.
- If results don’t seem to be accurate, call the lab to confirm how the process was executed. Human error can occur and with slim margins of error, precision is required.
Working Towards Establishing An Official AOAC Ethanol Testing Methodology Specific For Kombucha
On September 27, 2015, KBI president Hannah Crum and and Heath-Ade CEO Daina Trout, head of LGO committee (Special Projects Team) presented to the Stakeholder Panel on Strategic Food Analytical Methods (SPSFAM) at the annual conference of the Association of Official Analytical Chemists (AOAC) with the goal of establishing a new Working Group in order to develop a testing standard for the trace levels of ethanol in kombucha. Experts across various industries specializing in manufacturing, food chemistry, and laboratories were present as well as representatives from government organizations like the TTB.
The LGO team enumerated the difficulties with the testing methods used by regulators in the US. The message was clear: an accurate, standard method of testing that takes into consideration the complexities of kombucha such as the strands of living culture, yeast bodies, organic acids, low pH and other factors simply didn’t exist.
The stakeholder panel eagerly took up the process of formulating a Fitness for Purpose (FOP) statement. The FOP defines the parameters for the Working Group in order to vet methods submitted by scientists and firms from around the globe.
Fitness for Purpose statement: Methods need to accurately and precisely measure the ethanol concentrations to comply with alcohol and non-alcohol declarations in kombucha in-process and finished products.
Since AOAC standards are in use by governments around the world, including the US, the TTB (Tax & Trade Bureau) has been an active member of the Expert Review Panel (aka Working Group), including evaluating the submitted methods to ensure they meet the requirements put forth by the FOP.
Since 2015, the Expert Review Panel has met both in person as well as online in order to review the methods submitted. Sometimes the data provided was sufficient to move a method to First Action Status whereas other times additional data was requested and methods have been subject to re-review at later meetings. KBI President, Hannah Crum, has been present at every meeting to provide the industry’s perspective and to vote.
Methods approved into First Action Status are then used “in the wild” for 2 years. At that time, additional data demonstrating the method continues to satisfy the FOP is resubmitted for evaluation by the Working Group before being voted unanimously into Final Action Status. Once Final Action Status is achieved, the method is assigned an Official Method of Analysis number and published in the AOAC directory of methods (see below for more details).
Key Milestones Achieved
- KBI Attended Expert Review Panel on Ethanol Testing in Kombucha at AOAC 131’s Annual Meeting
- First Action Status Method Approved Sept 2016– GC-FID by NaturPro Labs STATUS UPDATE (2019): Currently under review by AOAC Statistical Committee
- First Action Status Method Approved Sept 2017 – Enzymatic Method by r-Biopharm STATUS UPDATE (2019): Data submitted to move from First Action to Final Action status
- KBI Awarded BEST WORKING GROUP OF THE YEAR 2017
- First Action Status Method Approved June 2019 – Headspace solid phase microextraction & GC-MS by MilliporeSigma
About the AOAC and The Official Methods of AnalysisSM (OMA) Program
The Official Methods of AnalysisSM (OMA) program is AOAC INTERNATIONAL’s premier methods program. The program evaluates chemistry, microbiology, and molecular biology methods. It also evaluates traditional benchtop methods, instrumental methods, and proprietary, commercial, and/or alternative methods. In 2011, AOAC augmented the Official MethodsSMprogram by including an approach to First Action Official MethodsSM status that relies on gathering the experts to develop voluntary consensus standards, followed by collective expert judgment of methods using the adopted standards. All methods in the AOAC Official MethodsSM program are now reviewed by Expert Review Panels for First Action AOAC Official Methods of AnalysisSM status, continuance, repeal, and/or to recommend for AOAC Final Action Official Methods status.
The OMA program has undergone a series of transitions in support of AOAC’s collaborations, evolving technology, and evolving technical requirements. Methods approved in this program have undergone rigorous scientific and systematic scrutiny such that analytical results by methods in the Official Methods of Analysis of AOAC INTERNATIONAL are deemed to be highly credible and defensible. The methods are published in the Official Methods of Analysis of AOAC INTERNATIONAL and supporting manuscripts are published in the Journal of AOAC INTERNATIONAL.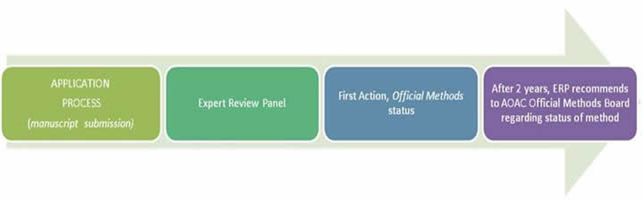
Why Traditional Ethanol Testing Methods Prove Inaccurate
Previously, kombucha has been subject to the same methods for testing ethanol as those for wine and beer, ferments that have been intensively studied for hundreds of years and offered commercially for thousands. The tools used for testing alcohol work very well with those ferments due to their higher alcohol content.
Unlike beer and wine, wherein most cases yeast are doing the fermenting, kombucha fermentation is two-fold and includes fermentation as well as oxidation. First, the yeast ferments sugar into ethanol and carbon dioxide. Then the bacteria feed on the ethanol and using oxygen, convert it to healthy organic acids (oxidation).
The acids produced by oxidation can have a similar density as alcohol, thus confounding the readings of “yeast only” attenuated equipment. In addition, raw, unfiltered kombuchas contain more particulate potentially confounding readings by causing light waves to refract incorrectly or shifting the gravity of the brew. The pairing of excess particulate and trace levels of ethanol in kombucha has made it difficult to use refractometers to obtain accurate ethanol readings.
Any testing method with a variance of +/- 1%, while typically would be considered very accurate, can mean a violation of labeling laws and trouble with local or federal inspectors and regulators. In 2010, in the US, this led to a voluntary withdrawal of all kombucha brands from the shelves of Whole Foods and other stores. As a result of the withdrawal, the kombucha industry lost millions in dollars in recalled product as well as the cost to reformulate, modify manufacturing procedures, revise labels, and institute tighter alcohol monitoring protocols. This event was the impetus behind the founding of KBI in order to unite brewers, establish best practices and develop strategies for protecting the industry.
Traditional Beer and Wine Testing Methods – May Be Suitable for In-House Ethanol Testing
Hydrometers and refractometers are inexpensive and easy to use pieces of equipment and kombucha brewers can benefit from the approximate alcohol readings they can provide for in house testing and quality control. Due to the very low alcohol content regulations, our industry requires finer controls and testing results to ensure compliance and these tools are not intended to be finely attenuated enough, even if kombucha specific scales are produced, to provide our members with confidence that their product will be in compliance.
Hydrometer
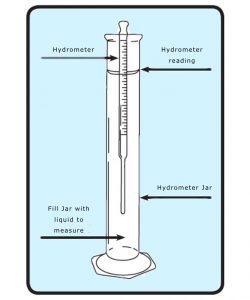
The hydrometer was invented by the female mathematician Hypatia in the 5th Century BC and measures the specific gravity, or density, of a liquid (how much solid is dissolved in the liquid to be exact). This is helpful in determining the creaminess of milk, for example, or the alcohol content of beer and wine, based on different scales that have been created for each specific substrate being tested.
Hydrometers are by no means exact in their measurements, however. The potential alcohol reading they provide is determined by applying a mathematical formula to specific gravity measurements taken prior to and directly following fermentation, which gives an estimate of the conversion of denser sugar into lighter alcohol. Samples must be maintained at a specific temperature for the reading to be accurate which in the case of kombucha means allowing it to warm up and thus potentially reactivating the fermentation process.
Applying the currently available scales for wine and beer to kombucha tests using this tool leads to inaccurate readings unless additional computations are taken.
Refractometer
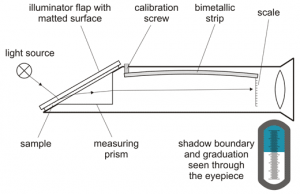
Another common tool used in the wine and beer industry is the refractometer, which can be digital or analog, and measures the angle of refraction of light when passed through the solution. That light reading is translated to sugar content via the Brix scale, which has an equivalent in terms of specific gravity. Like the hydrometer, the Brix is then measured pre and post-fermentation and the rate of change determines the potential alcohol content present. It also offers a rough measure of total sugars present in a brew.
Other Acceptable Methods for In-House Testing of Kombucha
Anton Paar – Near-Infrared Technology
One of the testing mechanisms that gained favor following the temporary market withdrawal of kombucha in 2010 is the Alcolyzer Beer Analyzing System manufactured by Anton Paar, a world leader in analyzing equipment. The Alcolyzer relies on Near Infrared Technology (NIR), which uses light waves at a known oscillation to pass through a particular substance. When it passes through at a specific frequency, it bounces back wavelengths that correspond to levels of potential alcohol. While this technology has been shown to be accurate for higher alcohol concentration products such as wine and beer, the amount of dissolved solids and the low amount of ethanol in kombucha in the past complicated the readings.
Anton Paar has recalibrated their Alcolyzer equipment to provide measurements that are specific to kombucha. Some of our producers comment that the results are now more accurate than the previous version.
OptiEnz Ethanol Sensor
The low level of ethanol required for non-alcoholic kombucha necessitates quick, affordable, and reliable testing that can be used to measure levels at or below 0.5% alcohol by volume (ABV). This study presents a comparison of four ethanol measurement technologies for the kombucha industry. Eight off-the-shelf kombucha products were analyzed in a blind test for ethanol content using gas chromatography (GC), an OptiEnz Sensors ethanol sensing system, an Anton-Paar Alcolyzer, and distillation paired with an Anton-Paar densitometer. Read the full study here.
Rare Combinations LLC Kombucha Alcohol Detector (KAD)
BABS compared the Rare Combinations LLC Kombucha Alcohol Detector (KAD) to gas chromatography paired with flame ionization detection (GC-FID) utilizing AOAC 2016.12. Standards were purchased from Cerilliant when applicable. Quality controls were made from grain alcohol and analytically proofed using a calibrated Anton-Paar DMA4500 densitometer that provides accurate density readings to five decimal places (± 0.00001). Five kombucha samples were purchased from a local grocery store and tested by GC-FID and KAD in duplicate to allow comparison. The KAD was operated according to instructions provided by Rare Combinations LLC. The ABV read-out and the raw value read‑out were recorded for each sample measured by the KAD. All sample measurements were performed in duplicate and occurred at room temperature (22-23~C). Read the full study here.
Rev.7.2.20

